Describe the Two Main Types of Chemical Bonds
A brief description of the different types of chemical bonds can be found below. The only pure covalent bonds occur between identical atoms.
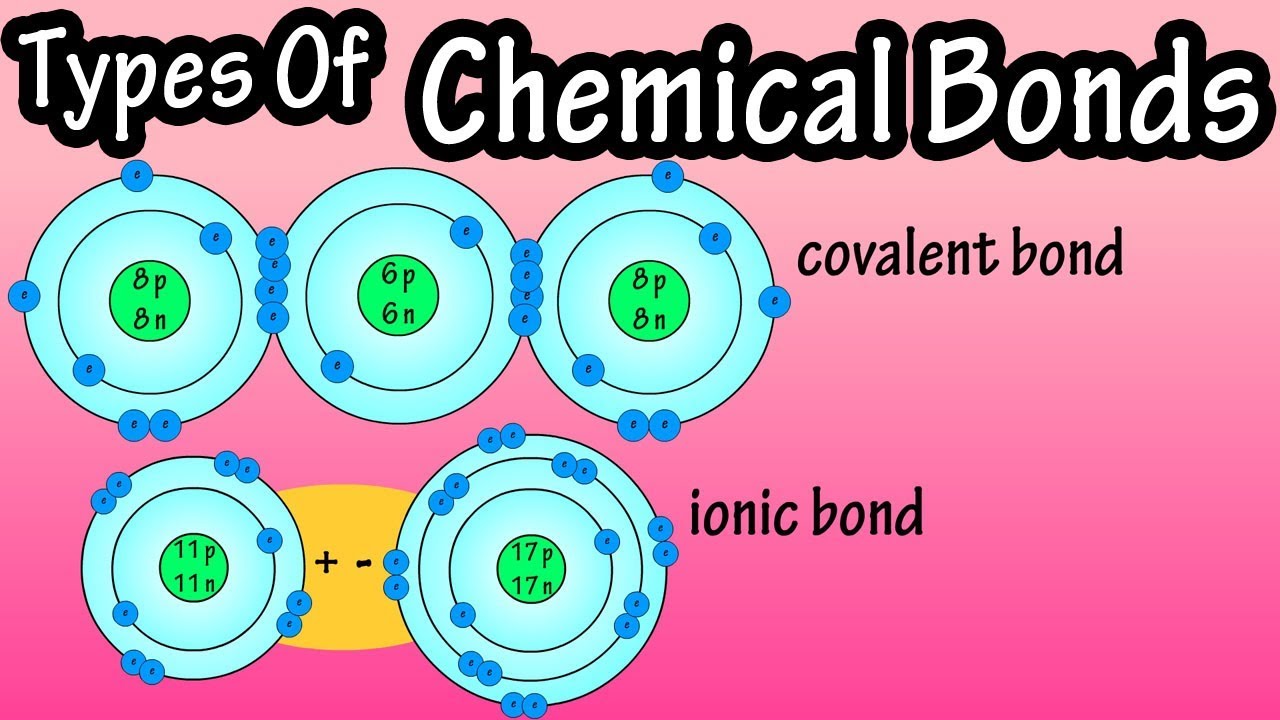
Types Of Chemical Bonds What Are Chemical Bonds Covalent Bonds And Ionic Bonds What Are Ions Youtube
When a molecule is made up of two or more atoms of different elements it is called a chemical compound.

. Hydrogen bonds refer specifically to the types of bonds formed with hydrogen atoms such as the water in sweat. Hydrogen bonds often abbreviated to H-bonds. There are 4 primary types of chemical bonds which are formed by atoms or molecules to yield compounds.
These bonds include both strong. If the substances are metal and non-metal then the bond between them is ionic. An ionic bond essentially donates an electron to the other atom participating in the bond while electrons in a covalent bond are shared equally between the atoms.
Given this bonding conditions ionic materials tend to be non-molecularwhereas covalent bonding can support discrete molecules. Ionic bonds form when metals and non-metals chemically react. For example an oxygen atom can bond with another oxygen atom to fill their outer shells.
There are four types of chemical bonds essential for life to exist. A double covalent bond is found in ethylene C 2 H 4 because two sets of valence electrons are shared. Chemical Bonding Study Guide.
The bond made by electron sharing is called a covalent bond. Ionic Covalent and Metallic. The type of chemical bond depends on the substances taking part in it.
The covalent bond can be categorised into the following categories based on the number of shared electron pairs. And thus there is electrostatic interaction between particles. Chemical bonds can be classified into two types as Primary strong bonds-.
Ionic bond is a chemical bond between atoms formed by the transfer of one or more electrons from one atom to the other. Finally for atoms with the largest. The metals form positively-charged ions and the non-metals form negatively-charged ions.
Covalent bonds also known as molecular bonds. Bonds formed between ions with opposite charges. Occur when there is a large difference in electronegativity.
Chemical bond refers to the forces holding atoms together to form molecules and solids. Chemical bonds are the forces of attraction that tie atoms together. Chemical bonds hold molecules together and create temporary connections that are essential to life.
Ionic bonds also known as electrovalent bonds. Learn vocabulary terms and more with flashcards games and other study tools. Hydrogen attracts and bonds to neighboring negative charges.
Generally when metals react with non-metals electrons are transferred from the metals to the non-metals. In this bond the electrons are. Bonds are formed when valence.
What are the three main types of bonds. The two main types of chemical bonds are ionic and covalent bonds. Key Takeaways Chemical bonds.
Polarized bonds form when two atoms share an electron but one of the elements has a stronger attraction to that shared electron. Chemical bonds are the connections between atoms in a molecule. Covalent bonding and covalent compounds will be discussed in Chapter 4 Covalent Bonding and Simple Molecular Compounds.
Two hydrogen atoms can share an electron to form the molecule H 2 and they are joined by a single covalent bond. Negative ions are formed by the gain of electrons. A more or less stable grouping of two or more atoms held together by chemical bonds is called a molecule.
The atom that gains an electrons is. Ionic covalent and hydrogen bonds. Usually there is some polarity polar covalent bond.
Polar covalent bonds also known as polar bonds. There exist four primary types of chemical bonds as listed below. A triple covalent bond.
There are three major types of chemical bonds. If two substances are non-metal then the bond is covalent. The bonded atoms may be of the same element as in the case of H2 which is called molecular hydrogen or hydrogen gas.
There are two types of covalent bonds. Start studying 3 main types of chemical bonds. These are the bonds that are strong and difficult to break.
These types of chemical bonds include. These types of bonds in chemical bonding are formed from the loss gain or sharing of electrons between two atomsmolecules. If two substances are metal then the bond between them is metallic.
This type of bond is also called the molecular bond. This force is of an electric nature and the attraction between electrons of one atom to the nucleus of another atom contributes to what is known as chemical bondsAlthough electrons of one atom repel electrons of another the repulsion is relatively small. A covalent bond is formed when two atoms with electronegativities share their electrons rather than trading them as happens in ionic bonds.
Chemical bonds allow all of the elements to combine in a variety of ways to create everything on Earth. Positive ions are formed by the loss of electrons. Types of chemical bonds including covalent ionic and hydrogen bonds and London dispersion forces.
Elements with large distances on the periodic table tend to form what bonds. Ionic bonds fall outside this umbrellaand are conceived to result from the TRANSFER of electrons between atoms to give discrete positive and negative ions. Consist of positive and negative ions.
Atoms bonded by sharing electrons. Occur when there is a small difference in electronegativity. Nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally.

Covalent Bond Definition Types And Examples
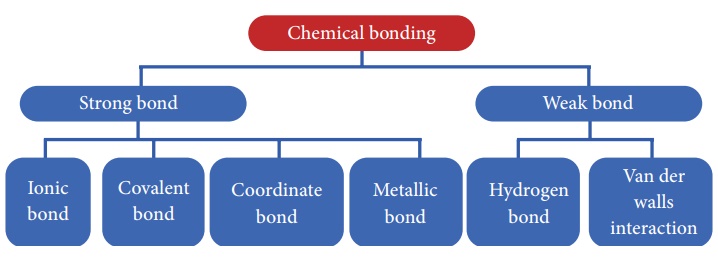
Types Of Chemical Bond Characteristics Formation Illustration

Comments
Post a Comment